HIV-1 pre-exposure prophylaxis (PrEP) relies on inhibition of HIV-1 replication steps. To understand how PrEP modulates the immunological environment, we derived the plasma proteomic profile of men receiving emtricitabine-tenofovir (FTC-TDF) or emtricitabine-tenofovir alafenamide (FTC-TAF) during the CHAPS trial in South Africa and Uganda (NCT03986970). The CHAPS trial randomized 144 participants to one control and 8 PrEP arms, differing by drug type, number of PrEP doses and timing from final PrEP dose to sampling. Blood was collected pre- and post-PrEP. The inflammatory profile of plasma samples was analyzed using Olink (N=92 proteins) and Luminex (N=33) and associated with plasma drug concentrations using mass spectrometry. The proteins whose levels changed most significantly from pre- to post-PrEP were CCL4, CCL3 and TNF-α; CCL4 was the key discriminator between pre- and post-PrEP samples. CCL4 and CCL3 levels were significantly increased in post-PrEP samples compared to control specimens. CCL4 was significantly correlated with FTC drug levels in plasma. Production of inflammatory chemokines CCL4 and CCL3 in response to short-term PrEP indicates the mobilization of ligands which potentially block virus attachment to CCR5 HIV-1 co-receptor. The significant correlation between CCL4 and FTC levels suggests that CCL4 increase is modulated as an inflammatory response to PrEP.
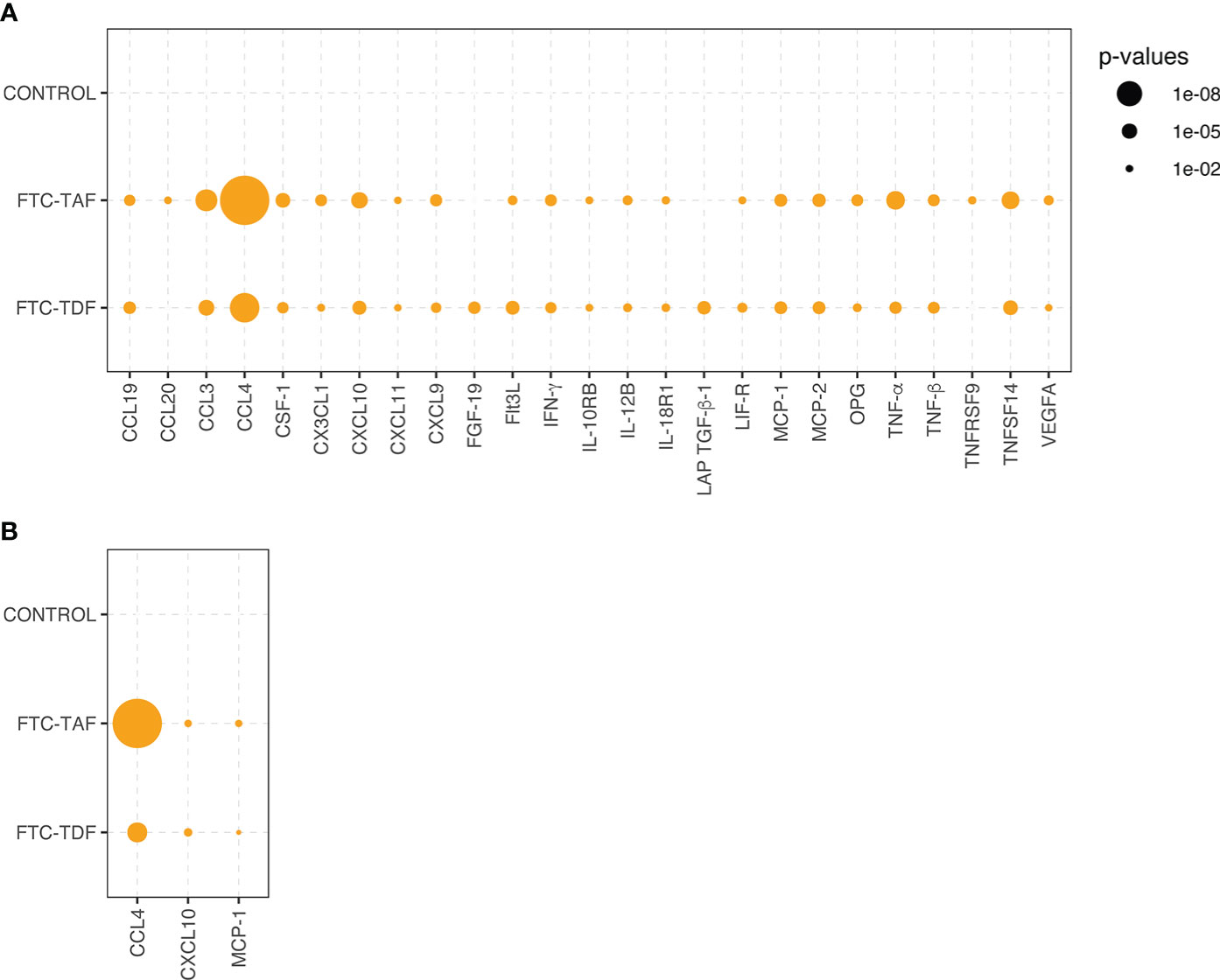
Statistical significance of inflammatory protein comparison between paired pre- and post-PrEP plasma samples. Statistical significance of comparative abundance values of all proteins detected in Olink (n=26) between pre- and post-PrEP samples from the two PrEP groups (FCT-TAF and FTC-TDF) are shown in (A) and for Luminex (n=3) in (B). Proteins which yielded a statistically significant difference (p < 0.01) are represented by circles whose size reflects the level of significance.
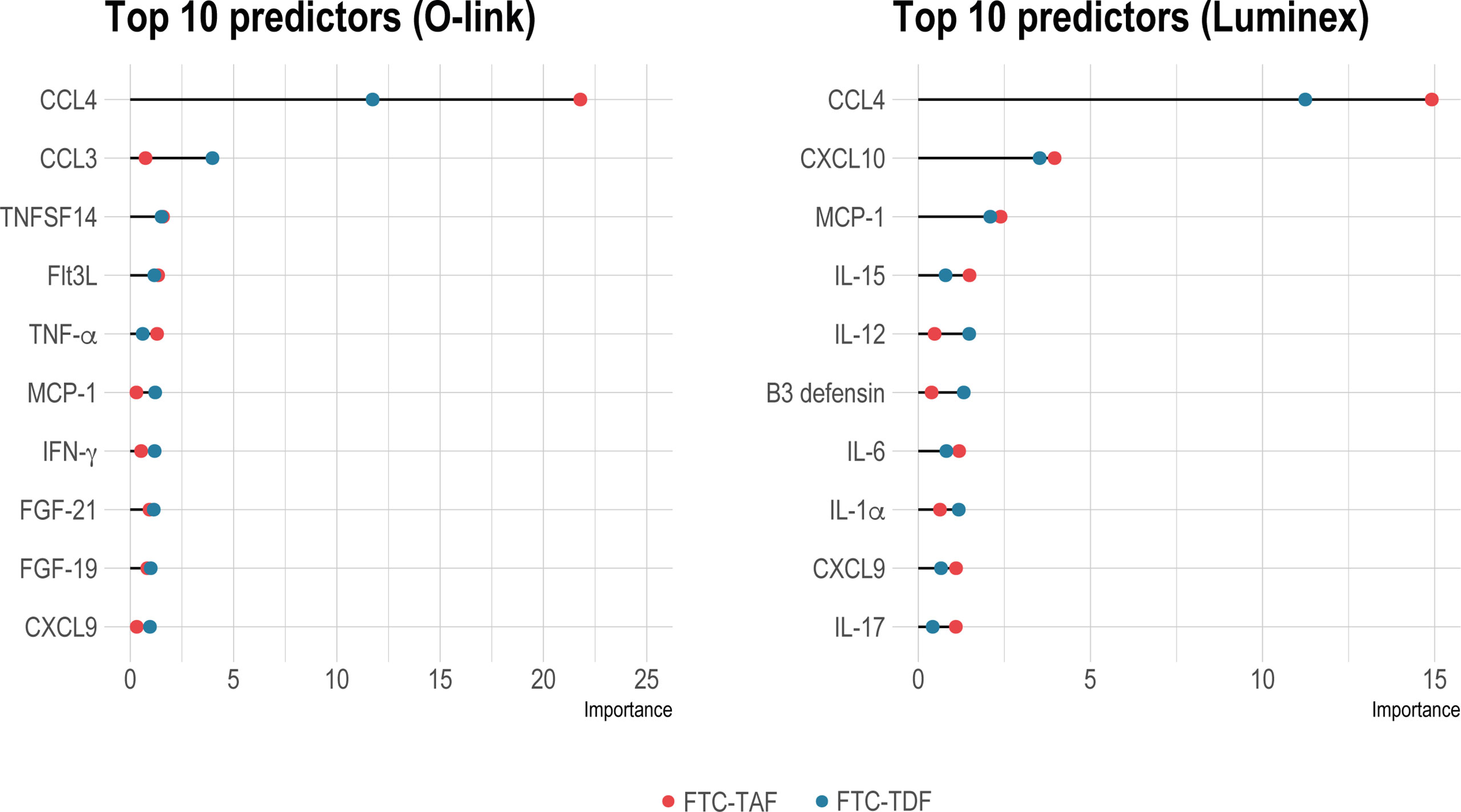
Identification of group status predictors by random forest analysis. Random forest analysis to reveal important predictors between pre- and post-PrEP groups in individuals receiving either FTC-TDF (blue dots) or FTC-TAF (red dots) is shown in on the left for Olink and on the right panel for Luminex. The top-ten significant predictors (p < 0.01) are shown.
Abundance level of proteins identified as significantly different between arms receiving FTC-TDF or FTC-TAF. PrEP arms were merged by the type of administered drug and compared between each other and to controls. Analyses of data is shown in (A) for Olink and (B) for Luminex. Results were considered significant when p < 0.05 *p < 0.05; **p < 0.01; ***p < 0.001.
Petkov S, Herrera C, Else L, Mugaba S, Namubiru P, Odoch G, Opoka D, Pillay AAP, Seiphetlo TB, Serwanga J, Ssemata AS, Kaleebu P, Webb EL, Khoo S, Lebina L, Gray CM, Martinson N, Fox J, Chiodi F. Mobilization of systemic CCL4 following HIV pre-exposure prophylaxis in young men in Africa. Front Immunol. 2022 Jul 27;13:965214. doi: 10.3389/fimmu.2022.965214. PMID: 35967369; PMCID: PMC9363563.